An electron orbital is mathematical function used in describing the wave-like mechanism of either an electron or many pairs of electrons in an atom. This function is later used when in need of providing the probability of locating an atomic electron in a specified area in the nucleus of the atom.
Explanation
Every orbital is shown by a number and a letter. the energy level in the electron orbital is shown by a number, for instance, 1 shows the energy level nearest to the nucleus, number 2 shows the next energy level of energy, 3 the next one, and so on.
Letters are there to refer to the shape of the orbital. These letters are s, p, d, f, g, h, I, j, and many more but here, we are only looking at letters p, s, and d and their corresponding shapes.
You should know that it is impossible to draw an orbital because an electron is capable of taking up all space but we can draw the shape that it takes up most of the time, presumably 90
THE s ORBITALS
Around the atomic nucleus, the s orbitals are found to be spherically symmetric like a very hollow ball with a nucleus at the center. The orbitals grow bigger as the energy levels increase and the electrons get located far away from the nucleus. The size of the nucleus grows in the order of 1s<2s<3s<4s and so on as shown by the figure below.
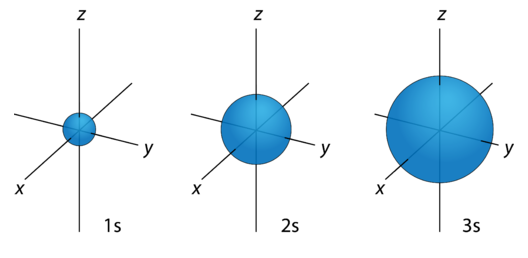
Now check the cross-section of the orbitals.
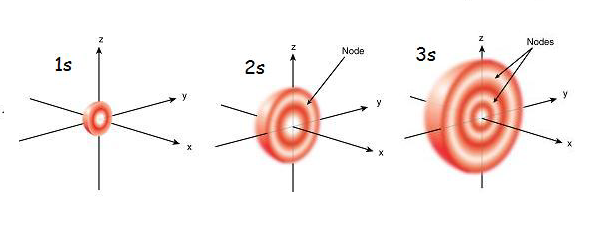
Under a careful look, you will see that the 1s orbital contains little density of electrons nearer the nucleus but as you get further from the nucleus it builds up to the optimum. When it gets at the furthest point electron density starts to decrease rapidly.
When you consider a 2s orbital you will notice that it has similarities with the 1s orbital. The only difference is that it contains a symmetrical sphere of electron density on the inside of the outer sphere. Much like putting a tennis ball inside another one. Between these two electrons, there is a surface that has no probability of locating an electron. It is called a nodal surface or simply a node.
The 3s orbital is much larger than the first two and has three nodal surfaces.
P ORBITALS
You should know that not every electrons are found in s orbitals. The only orbitals to be located at the first energy level is the 1s orbital. However, the 2p orbitals are found in the second energy level together with the 2s orbitals.
The difference between a 2s orbital and a 2p orbital is that a p orbital points in a certain direction while an s orbital points at no particular direction. See the diagram below.
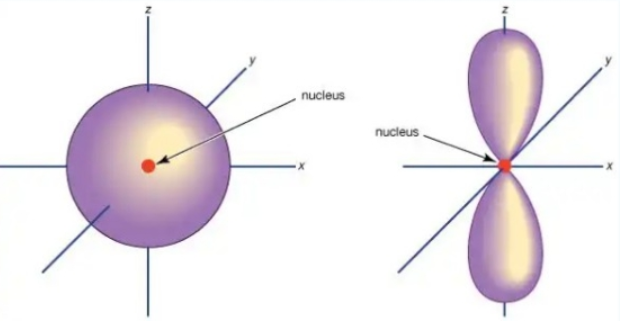
In any energy level, three equivalent p orbitals are found and points at right angles to each other. They are shown by symbols px, py and pz. This does not primarily mean that the symbols sticks because the orbitals are constantly changing directions as the atom moves. You should note that the p orbitals in the second energy level are named 2px, 2py and 2pz and also 3py, 3pz and 3pz and so on.
Nb, all levels have p orbitals in exception of the first level.
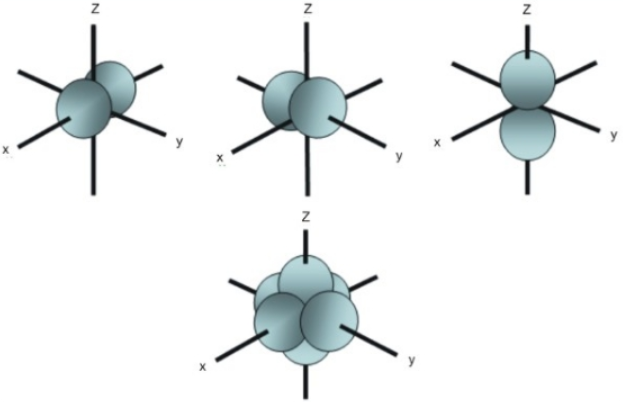
THE d ORBITALS
In the third energy level, five d orbitals are present. They have complicated names and shapes. The 3s and 3p (3px, 3py 3px) are present too. A total of nine orbitals are found in the third energy level. The five 3d orbitals are named; 2dxy, 3dxz, 3dyz, 3d(x-y)^2 and 3dz^2.
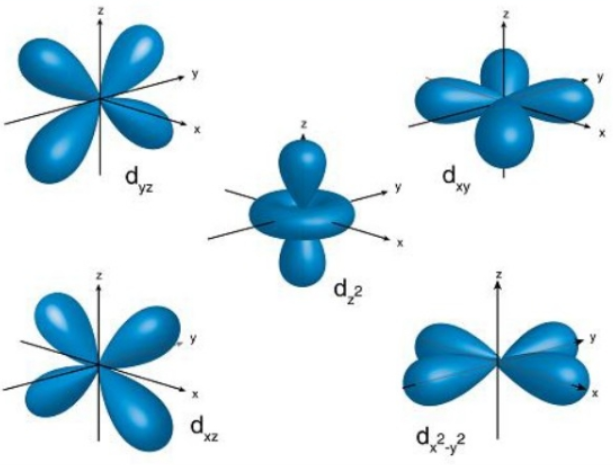
The manes of the 3d orbitals are only understood when [put into groups. Group 1 has the 3dxy, 3dxz, and 3dyz orbitals while the rest belongs to the second group. These names say that the orbitals lie in planes x-y, x-z, and y-z respectively. Every orbital contains four lobes and each lobe points in the middle of the two axes and not along the axes. Group 2 has the 2d9x-y)^2 and 3dz^2. The lobes of the two orbitals point along the axes.
The 3d(x-y)^2 orbital are similar to group 1 in exception to that instead of the lobes pointing between the axes they point along the x and y axes.
The 3dz^2 orbitals are similar to a doughnut wearing p orbital.