A solid solution is defined as a mixture of 2 solids of crystalline nature which together exist in the form of a crystal lattice or new crystalline solid.
The mixture can be made efficient by combining 2 solids when in liquids, they are melted at a higher temperature and afterward, by the cooling effect, it results in the creation of a new solid or by vapor deposition of starting materials on the top of substrates to build thin films.
Like with fluid, solid substances consist of different degrees belonging to mutual solubility, which depend on their crystalline structure and chemical properties, which helps in determining that in what manner their atoms suitably together fit into a mixed lattice of the crystal.
Mixed lattice has the possibility of being substitutional, where the atom of a starting crystal replaces interstitial or other crystal, in which an atom generally occupies the position present vacant or empty in a lattice.
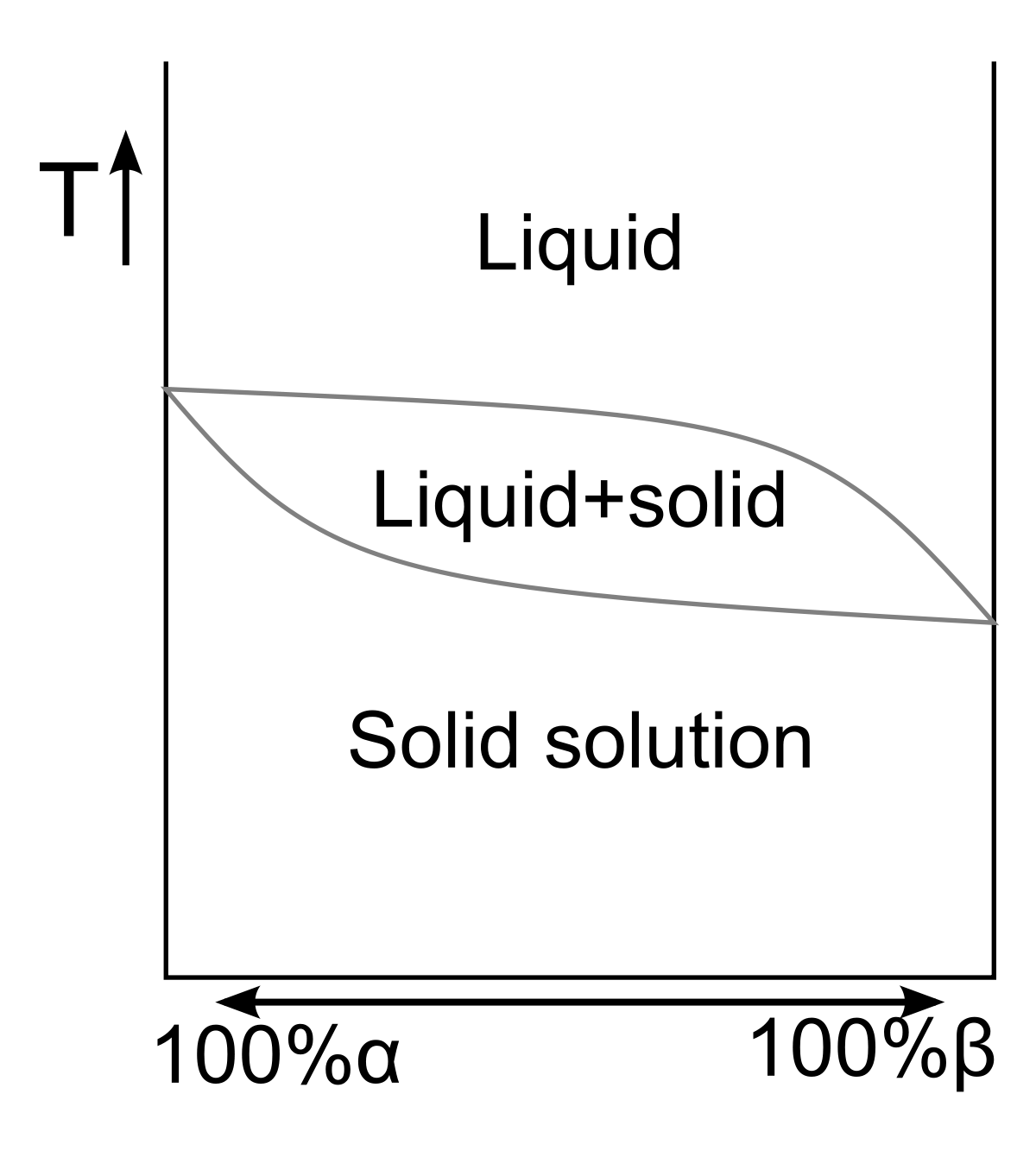
Substances can be soluble on a partial basis or also, on a completely comparative range of concentrations, generating the crystal, the properties of whose, continuously vary regarding range. So, it provides a method for tailoring solid solution’s properties for particular applications.
There are numerous solid solutions which in nature appears, in minerals’ form, created in the conditions of pressure and heat.
An example: The Olivin Mineral Group, specifically the forsterite-fayalite series, the series, the members of whose vary to fayalite (Fe2SiO4) from forsterite (Mg2SiO4).
2 compounds having same crystal structures and these compounds form the substitutional solid solution which ranges from (Mg) Magnesium present 100 percent to Iron (Fe) present in 100 percent, which includes all the proportions, including physical properties, which differ smoothly right from forsterite to fayalite.
Semiconductors’ solid solutions are having great technical value, as is the combo of (GaAs) gallium arsenide with (InAs) indium arsenide, (AlAs) aluminum arsenide, or (GaP) gallium phosphide.
Solid substances’ properties can be leaned to values between end compounds by modifying or adjusting the comparative/relative proportions of compounds; let’s say, the band gap or the energy gap for the combinations of GaAs and InAs can by anywhere set between the value for pure GaAs (1.4 eV), and for pure InAs (0.36 electron volt [eV]), with comparable changes in the optical and electrical properties of the material.
This flexibility type makes solutions of semiconductor solid extremely beneficial for various types of optical and electronic devices, which includes solar cells, transistors, light-emitting diodes (LEDs), infrared detectors, and semiconductor lasers.