p-Block Elements: Coordination compounds
Structural and stereoisomerism
Compounds that have the chemical formula but can be represented in different structures are known as isomers. Variant isomerism types occur because of complicated formulas of coordination compounds, the variety of bond types and the number of shapes possible. These are described below.
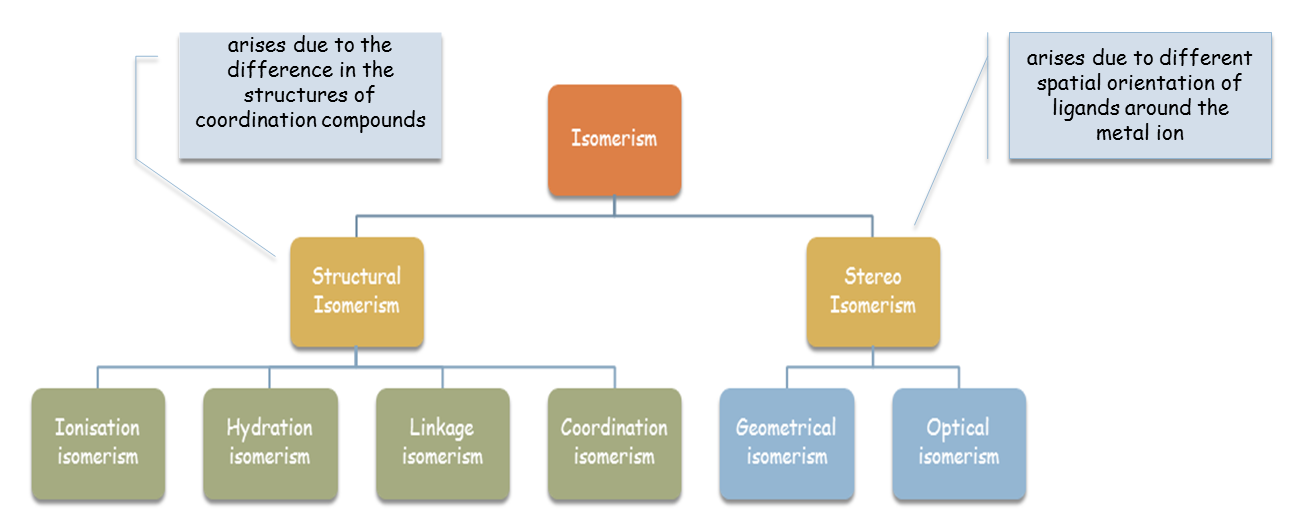
Structural isomerism
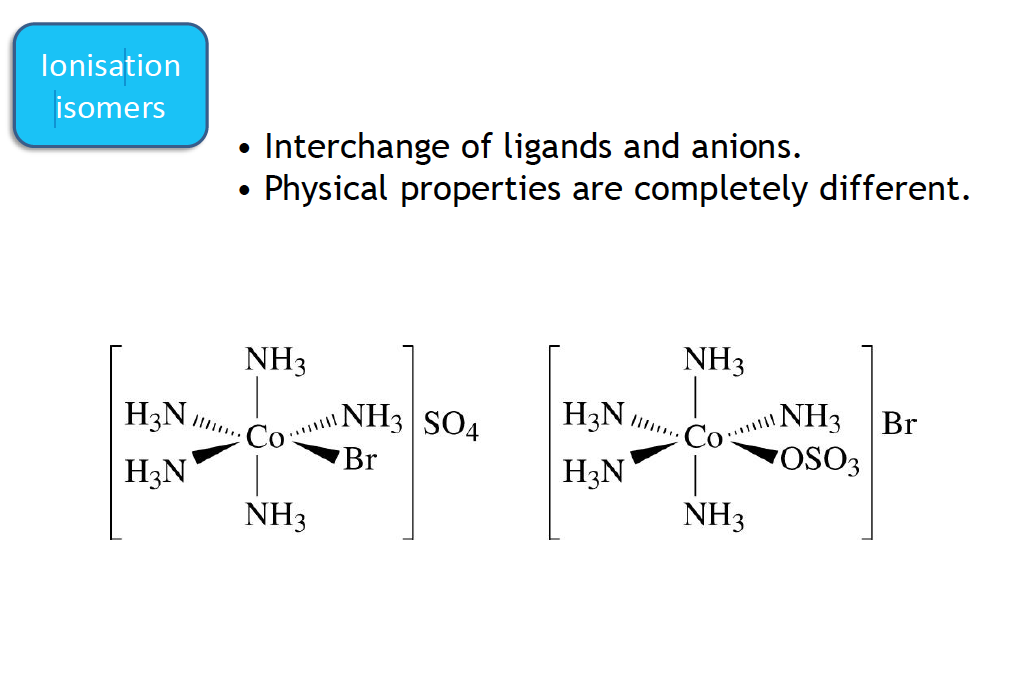
Ionisation isomerism
This type of isomerism arises due to the difference in the position of groups being inside or out the coordination sphere of the complex, e.g.[Co(NH3)5Br]SO4 (complex 1). and [Co(NH3)5SO4]Br (complex 2).There is interexchange of Br and SO4 in the isomers and since the two isomers on ionisation give different ions these are called ionisation isomers. Complex 1 on ionisation will give rise to [Co(NH3)5Br]2+ and SO42- ions while complex 2 on ionisation will give rise to [Co(NH3)5SO4] + and Br– ions. Another pair of complex exhibiting this type of isomerism is [Co(NH3)5Cl2]NO2 and [Co(NH3)5(Cl)(NO2)]Cl.
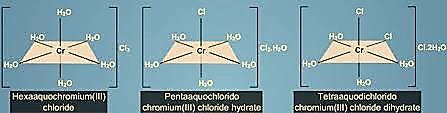
Hydration isomerism
This type of isomerism is similar to ionisation isomerism. This isomerism is also known as ‘hydrate’ or ‘solvate’ isomerism when arises due to the replacement of a coordinated group by the water of hydration. An example to illustrate this type of isomerism is: [Cr(H2O)4Cl2]Cl.2H2O, [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)6]Cl3. These are the 3 isomers that differ on a big scale from one to another in their physical and also in their chemical properties. While the coordination number of chromium is 6 in all the three cases, the isomers contain a different number of coordinated water molecules.
Coordination isomerism
This type of isomerism is observed in compounds with both, anionic and cationic parts of the coordination compound that are complex in nature. Consider the following example of [Co(NH3)6][Cr(CN)6], in which the NH3is bound to Co3+ and the CN- to Cr3+ and [Co(CN)6][Cr(NH3)6. In this case, NH3 are bound to Cr3+ and the CN– to Co3+, both the anionic and cationic parts are complex ions.
Linkage isomerism
Linkage isomerism arises in coordination compounds that have ambidentate ligand. For example, in NO2 – the nitrogen, and an oxygen atom, can both donate their lone pair. An example to illustrate this type of isomerism is :[Co(NH3)5(NO2)] and [Co(NH3)5(ONO)]. In the first complex, the coordination occurs through the nitrogen of the NO2 group. In the second complex, the coordination occurs through oxygen.
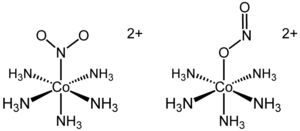
Stereo Isomerism
Geometrical isomerism
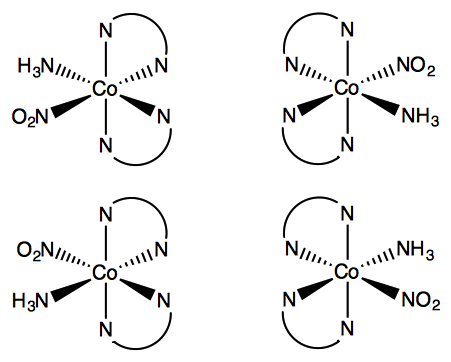
From the octahedral complexes of the types Ma6, Ma5b, Ma4b2, Ma3b3, only Ma4b2and Ma3b3, show two isomeric forms. In an octahedral (Ma2b4) complex (coordination number 6, a and b are unidentate ligands) there are two different positions that ligands can occupy relative to one another. In the two structures below it can be seen that the two ammonia ligands could be 90° or 180° relative to one another. This gives a cis- isomer and a trans- isomer. This type of isomerism also arises when bidentate ligands such as NH2CH2CH2NH2 (en) are present in the complex, e.g. [CoCl2(en)2]
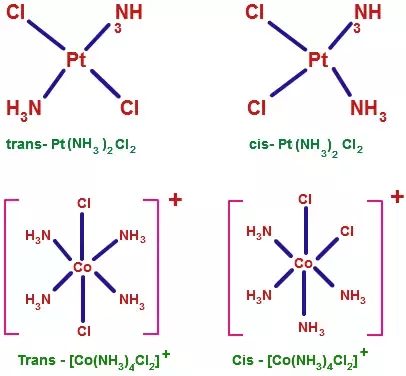
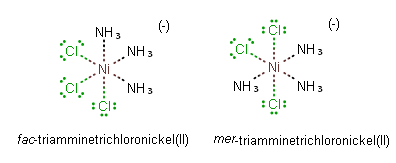
Among the complexes of the type Ma3b3 in an octahedral geometry, another type of isomerism is observed in which the isomers are known as facial (fac) and meridional (mer) isomers. If the three ligands ‘a’, or ‘b’, are located on the same plane, it forms a meridional (mer) isomer. However, if these ligands are adjacent to each other and form a triangular face of the octahedron, then it gives a facial (fac) isomer. For coordination number 4, only square planar geometry exhibits geometrical isomerism. It is exhibited by complexes of type Ma2b2,Ma2bc,Mabcd. In square planar Ma2b2, the two ligands ‘a’ may be arranged adjacent to each other, in a cis isomer, or opposite to each other, in a trans isomer. Geometrical isomerism is not observed for complexes with lower coordination number.
Optical isomerism
This type of isomerism can be exhibited with certain ligands such as an octahedral complex ion.It does not relate to the idea of a central carbon atom surrounded by four different groups, as in the case of organic chemistry. However, it considers two molecules that are not superimposable onto each other. Such types of molecules are known as enantiomers.