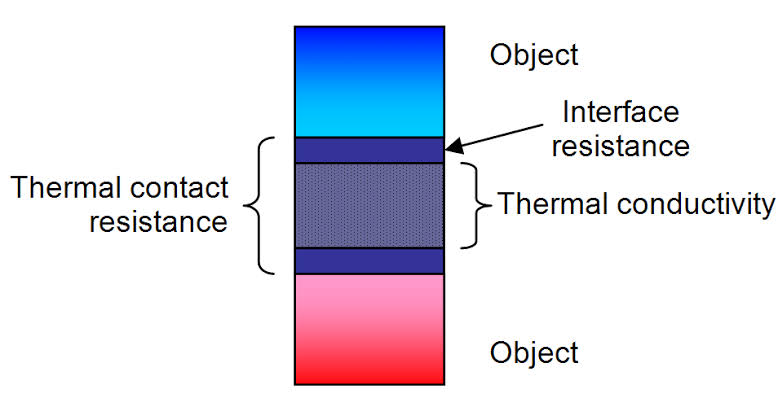
Thermal conduction simply defined as the transport of energy due to random molecular motion across a temperature gradient somehow is distinguished from energy transport by convection and molecular work but in that, it does not involve the macroscopic flows. Mainly in engineering practice, it is common to work in term of qualities which are derivative to thermal conductivity which can be implicitly taken into account. In simple words, the thermal conductivity of a material is a measure of its ability to conduct heat. Then heat transfer occurs at a lower rate in materials of low thermal conductivity and metals have high thermal conductivity and very efficient at conducting heat while insulating materials are totally different in this perspective.
What are the influencing factors for thermal conductivity?
There are so many factors that influence thermal conductivity such as temperature, chemical phase, thermal anisotropy, electrical conductivity, magnetic field, and isotopic purity. Firstly the effect of temperature on metals and non-metal is totally different from thermal conductivity. If pure metals are taken into consideration then in this electrical conductivity decreases with increasing temperature and thermal conductivity the product of two will remain constant but when the temperature becomes absolute zero then thermal conductivity sharply decreases. Then there is a chemical phase in which a material undergoes a change in a phase like from liquid to solid or to the gas then the thermal conductivity changes abruptly. When freely moving valence electrons transfer not only electric current but also heat energy by which thermal conductivity tracks electrical conductivity. Thermal Hall effect or right reduce effect is the name given to the magnetic field on thermal conductivity. Isotopic purity has a direct dependence on the thermal conductivity of a crystal.
What is the theoretical prediction?
The atomic mechanism, particularly of thermal conductivity, may vary from different materials and such as thermal conductivity is difficult to predict from the first principle. Thermal conductivity in gas is mediated by discrete molecular collisions as it is a simplified picture of solid this conductivity can be occurred by two methods first is the migration of free electrons and second is lattice vibrations and the first mechanism dominates in pure metals and the second mechanism dominates in non – metallic solids where thermal conductivity in liquids are poorly understood by the contrast of microscopic mechanism and also there is no molecular picture which is both simple and accurate.