Alcohols have various uses in our daily lives, and in this article, some uses of ethanol and methanol will be discussed.
Uses of Methanol
Mainly methanol is used in organic synthesis as a solvent, antifreeze, and as fuel. It has toxic properties and it is frequently used as a denaturant additive for the manufactured ethanol used for the industrial purpose. Frequently it is called the wood alcohol, as once it was primarily produced by the destructive distillation of wood and it was obtained as the byproduct. This is also used for the production of biodiesel by the transesterification reaction. It is a common solvent in the laboratory and especially useful for the UV/VIS spectroscopy and HPLC, as it has low UV cutoff.
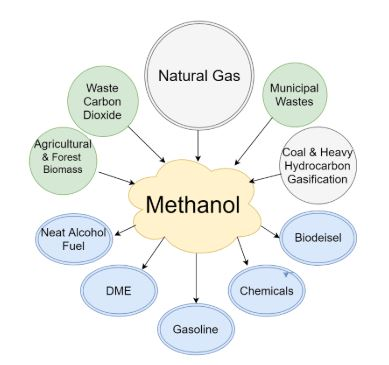
It is largely used for the manufacturing of other chemicals. Almost 40% of the produced methanol is converted to the formaldehyde and then it is used to make the various products such as the plywood, paints, plastics, permanent press textiles, and the explosives. Methanol that is produced from the organic compounds or the wood is an excellent renewable alternative to the petroleum-based hydrocarbons. With the proper use of the corrosion inhibitors and cosolvents, low levels of the methanol can be used in the existing vehicles. Many racing classes including the mud racers and the drag racers use the methanol as the primary source of fuel.
Uses of Ethanol
Ethanol is an alcohol that is obtained by the fermentation of starches and sugars and by chemical synthesis. For alcoholic beverages, it is an intoxicating ingredient and is also being used as a solvent in the explosives and as an additive for the replacement in the petroleum-based fuels. It is the most common and the oldest recreational drug which causes intoxication characteristic drunkenness and its consumption in sufficient amounts causes the neurotoxicity. It is widely used as fuel, as a solvent and as feed stock for the synthesis of the various chemicals.
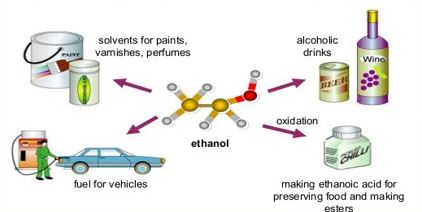
It has many minors uses as well. It is also used as a medical wipe and is being widely used as an antiseptic in the antibacterial hand sanitizer gels. Ethanol dissolves the lipids and denature the proteins and eventually kills the organisms including fungi, bacteria, and many viruses. At present ethanol is used for the preparation of more than 700 liquids including iron supplements, mannitol, acetaminophen, furosemide, sulphamethoxazole, and many other products. Its largest single use is as the fuel additive in the fuel engine. It has also been used as rocket fuel. Its solubility in water is high and is an ideal solvent for many products.