Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular the formula of phenols is C6H5O6. The synthesis of Phenols is Natural as well as artificial.
Uses of phenol
Phenols are compounds with a hydroxyl group attached to the benzene ring. The hydroxyl group thus attached is electron deficient due to the sp2 hybridized atom to which the OH group is attached. This is because of the high electronegativity of the sp2 hybridized carbon of the benzene ring.
The electronegativity of phenols can be further increased or decreased by the attachments of the electron withdrawing or the electron drawing groups subsequently. The electron withdrawing groups increases the electronegativity of the phenols. Whereas, the electron donating groups decrease the electronegativity of phenol. It’s a complete matter of how to stabilize the negative charge generated in the phenoxide ion.
Phenols have many industrial uses. This is because the ease with which it could be converted to phenoxide ion. As a result, the phenols are synthesized on quite a large scale since the 19th century.
Due to its wide applications as a reagent, more than two third of the phenols produced is used in the manufacturing process of plastics as a reagent. In today’s world, one can not live without the use of plastics in their day to day lives. We are surrounded by plastic from all around ourselves. The condensation reaction of phenol with acetone results into bisphenol A. The bisphenol A compound is used on a major scale in polymer industries to synthesis several epoxide resins and polycarbonates.
Phenol is used extensively in industries in manufacturing of phenol resins. In the making of phenolic resins, the phenol plays a major role. This is because of the polarization reaction between the phenol and the formaldehyde compounds. The phenolic resins are famously named as the Bakelite. Bakelite is used extensively in many industries. It has a high capacity for withstanding heat and resistance to other chemicals. As a result, Bakelite is used majorly in the making of switches and other electrical materials.
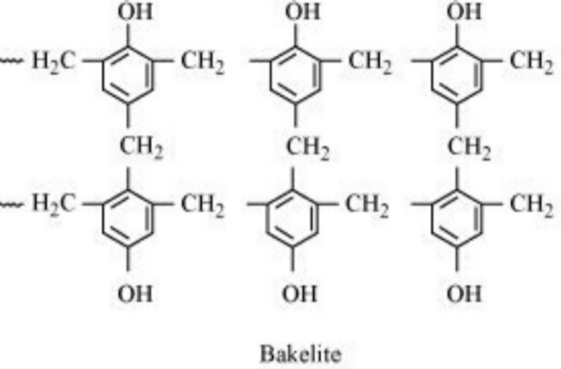
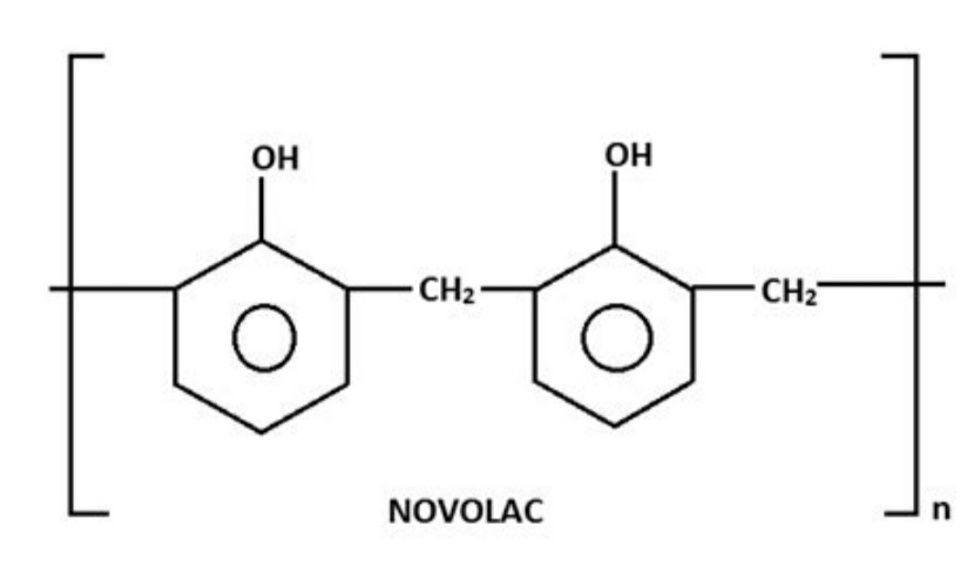
Novolac is another material produced in the intermediate step of the making of Bakelite. Novolac is used as adhesives and for binding purposes in many industries. It is another material where there is the use of phenols.
Another use of phenols include molecular biology. It is in major research for the process of extraction of nucleic acid from tissue samples.
Cosmetic industries also make use of phenols on a large scale. The manufacturing of sunscreens, hair colours and skin lightening creams have extensive use of phenol compound.