The model of Valence Shell Electron Pair Replication is oftentimes abbreviated as VSEPR (known as “Vesper”). It is fundamentally a model which predicts a molecule’s geometry.
In particular, VSEPR models see the molecular geometry and bonding of polyatomic ions and organic molecules. It is beneficial for almost the whole of the compounds that possess a central atom which is not metal.
Importance of VSEPR Models
- Lewis structures solely indicate the types and number of bonds in between atoms, because they are restricted to two dimensions. The 3D shape of ions and molecules is what model of VSEPR predicts but is inefficient to provide any particular information which is related to bond or bond length.
- The basis of VSEPR models are on the theory that electrons on all sides of a central atom will set up themselves to reduce repulsion, and it determines the “geometry of the molecule”.
- Also, VSEPR models can predict the shape of almost all compounds, which consist of a central atom, considering the central atom is not metal. Each shape features a name and the ideal bond angle correlated with it.
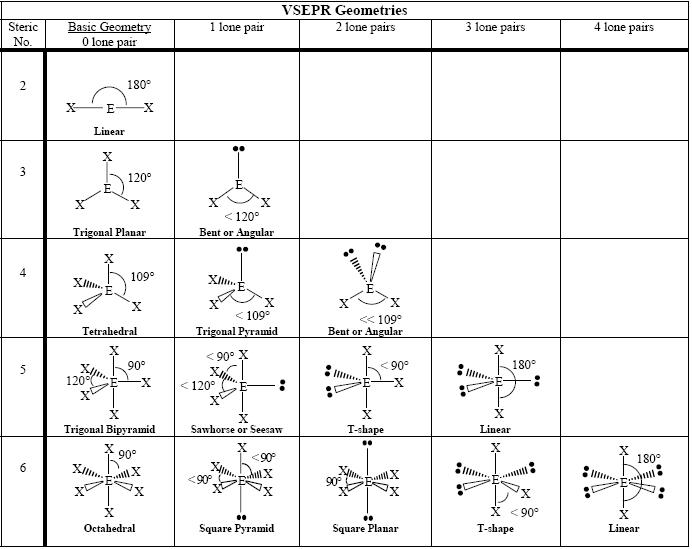
The major difference between the geometry of electron pair and “molecular geometry” is that molecular geometry doesn’t involve unpaired electrons, on the other side, “electron pair geometry” involves both unpaired electrons and bonded atoms.
In case there are no unpaired electrons in a compound being determined, then the geometry of both molecular and electron pair will be the same.
Molecular Geometry
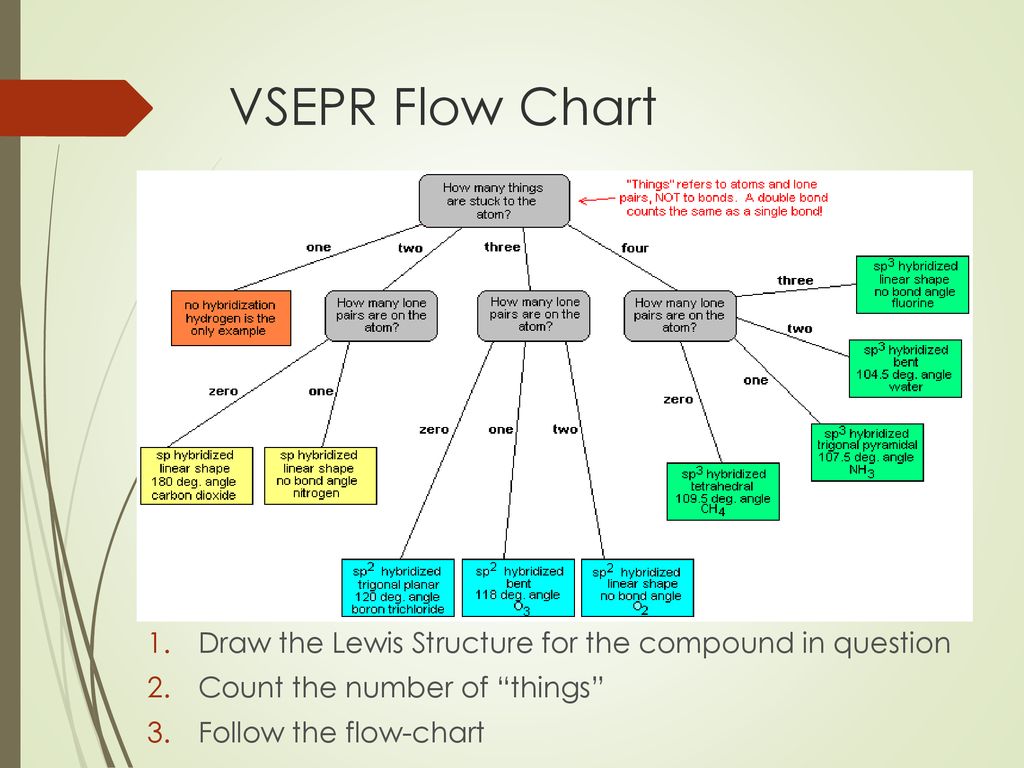
Steps to Use VSEPR
- Draw Lewis structure for a molecule or an ion in question.
- Find out the number of electron groups around a central atom. Each electrons’ lone pair is counted as a solo group. Each bond is counted as a single group, even though it’s a double or a triple bond. Find the equivalent electron geometry from periodic table.
- Find out the number of bonding and lone pairs around a central atom, and use it to find molecular geometry.
VSEPR Notation
The notation of VSEPR presents a general formula for chemical species classification which is based on electrons pair’s number around the central atom. However, it’s important to note that not all chemical species consist of exactly similar molecular geometry. Let’s say, sulfur dioxide (SO2) and carbon dioxide (CO2), are both species, however, one is bent and the other one is linear.
Occasionally, a notation is extended to combine “lone pair electrons”. This can be misleading as water can be pointed out as a chemical species which depends on text or author opts. In general,
- A is represented as a central atom.
- The representation of a number of atoms being bonded to a central atom is used by B or X.
- Lone pairs number is represented by E on a central atom (which excludes lone pairs on bonded atoms)
Limitations of the VSEPR theory
- The VSEPR model is not just a concept. It doesn’t attempt to explain or explain any predictions or observations. Comparatively, it’s an algorithm which precisely predicts the structures of a considerable statistic of compounds.
- VSEPR is easy and advantageous but doesn’t work out for whole chemical species.
- Firstly, the “idealized bond angle” doesn’t always match the calculated values. Let’s say, VSEPR is assured of and predicts that it have the identical bond angles, but the structural study has shown that the bonds in 2 molecules continue to be different by an angle of 12 degrees.
- Model of VSEPR even predicts that when halides of group-2 are actually bent, they’ll be linear. When VSEPR is insufficient, atomic orbitals and quantum mechanics can result in higher sophisticated predictions.