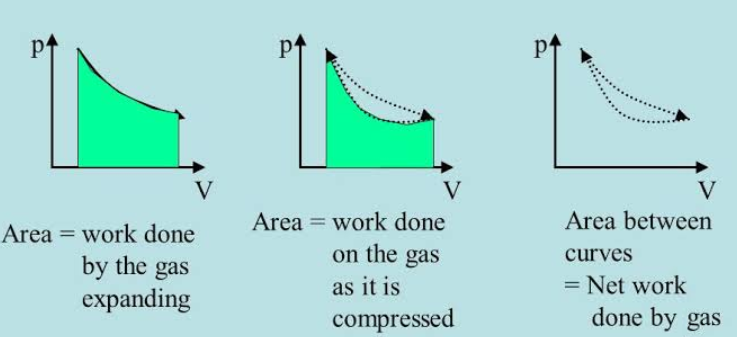
Work done by a gas
Gases can work through enlargement or compression against relentless external pressure. Compressing of a gas is also known is the isothermal process. Work done by gases is additionally generally known as pressure-volume or PV.
Let’s consider gas is contained in a piston. Two tubes leading to the piston can let gas in and out of the piston to control the movement of the piston. Energy is added to the gas molecules in case the gas becomes hested. We can observe the rise in average K.E. of the molecules by measurement however the temperature of the gas will increase. As the gas molecules move quicker, they also collide with the piston more often. These progressively more frequent collisions transfer energy to the piston and move it against external pressure, increasing the overall volume of the gas. In this example, the gas worked on the surroundings, which includes the piston and also the rest of the universe.
To calculate how much work a gas has done or it’s been done to it against a continuous external pressure, we use a variation on the previous equation: where P external is that the external pressure (as opposed to the pressure of the gas within the system) and ΔV is that the modification within the volume of the gas, that can be calculated from the very first and final volume of the gas:
ΔV = V final − V initial
Since work is energy, it has units of Joules (where 1J = 1 kilogram. m^2 \ s^2) you may also see other units used, such as atmospheres for pressure and liters for volume, resulting in L. atm as the unit for work. We can convert L ⋅ atm to convert to Joules using the conversion factor of 101.325 J / 1L⋅atm.
As a matter of convention, negative work happens whenever a system does work on their surroundings.When the gas does work the volume of a gas increases and the work done is negative.When work is done on the gas, the volume of the gas decreases and work is positive.When the gas is compressed, energy is transferred to the gas so the energy of the gas increases due to positive work.
Calculating Work Done on a Gas
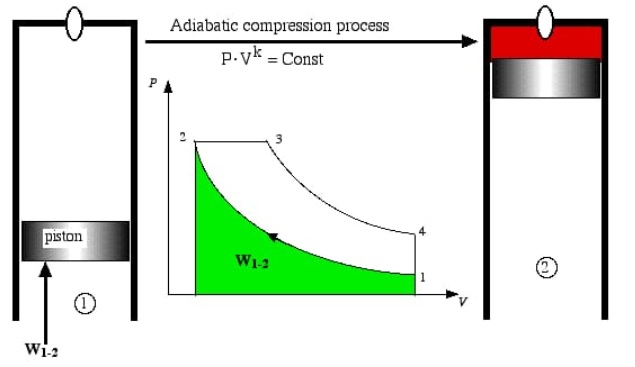
To illustrate a way to use the equation for PV work, we can imagine a bicycle pump. We will assume that the air within the pump can be approximated as a perfect gas in a piston. We can work on the air in the pump by compressing it. Initially, the gas contains a volume of 3.00 L. We apply a constant external pressure of 1.10 atm, to push down the handle of the bike pump until the gas is compressed to a volume of 2.50 L. How much work was done on the gas?
We can use the equation from the last section to calculate that how much work has been done to compress the gas:
w = −Pexternal × ΔV
= −Pexternal × (Vfinal −Vinitial)
If we plug in the values for P external, V final and V initial for our example, we get:
w = −1.10atm × (2.50L− 3.00L)
= −1.10atm × −0.50L
= 0.55L ⋅ atm
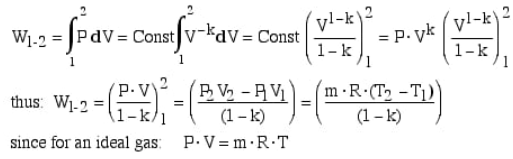
We know that work was done on the gas since the level of the gas decreased. That means the worth of work we have calculated ought to be positive, which matches our result. We can additionally convert our calculated work to Joules using the conversion factor. Thus, we did 56 J of work to compress the gas in the bicycle pump from 3.00 L to 2.50 L.